News
News
PublISHED ON
UpDATED ON
Just over 75 per cent of clinical studies registered after one year
As from 2022, all clinical studies funded by the Swedish Research Council shall be registered in a public database. A recent follow-up shows that the majority of the clinical studies awarded funding in 2022 have been registered.
All research involving humans must be registered before it starts.
“Strictly speaking, our guidelines for study registration only clarify what researchers who comply with good research practice are already expected to do. Ensuring all clinical studies are registered is important for many reasons, not least to promote effective research collaborations and to reduce the risk of repeating research unnecessarily,” says Jonas Oldgren, Secretary General for Clinical Research at the Swedish Research Council.
The guidelines are complied with by the majority
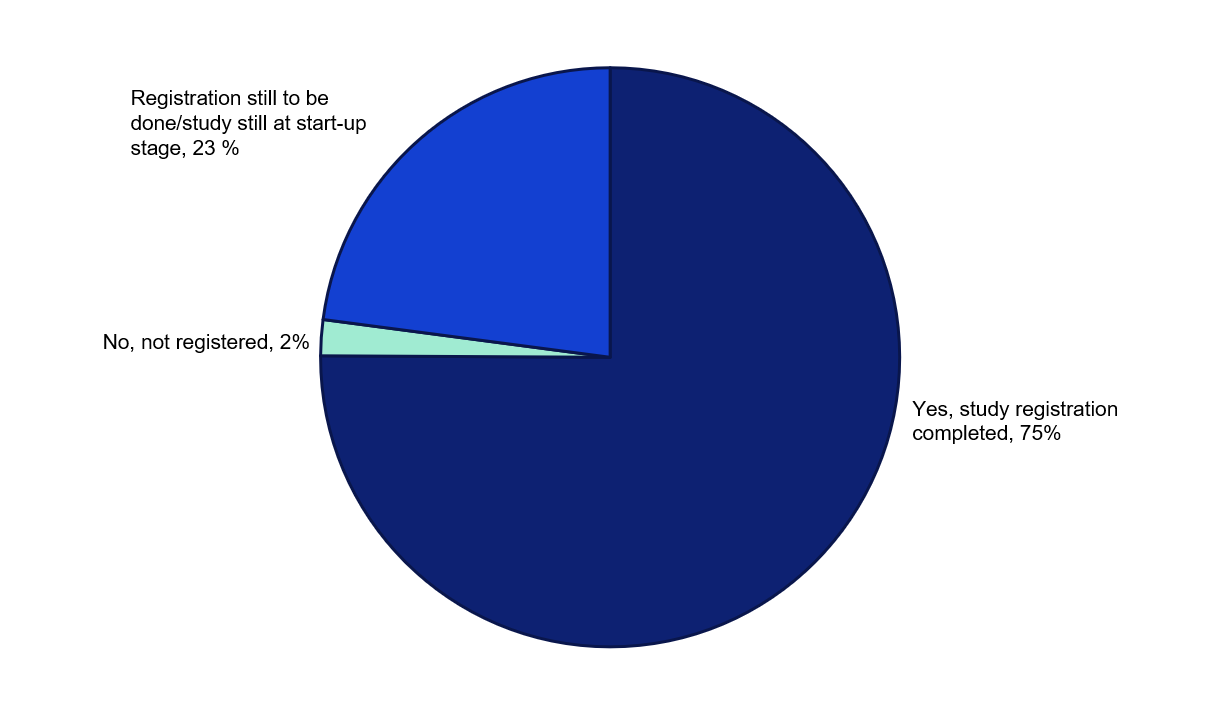
For 75 per cent of the studies awarded funding in 2022, the grant recipients report that the studies are registered and have started. 23 per cent state that they are still at the planning or start-up stage, or alternatively that study registration will be done. 2 per cent of the studies have not yet been registered, despite reportedly having started.
“It is gratifying that the majority are following our guidelines, but the goal is of course that all clinical studies shall be registered, irrespective of who is responsible for the study, or who funds the research. To close the circle, it is also important that all completed clinical studies are reported and published, so that the results actually do reach out and create benefit. This, of course, applies also to those clinical studies that do not show results according to the original hypothesis,” says Jonas Oldgren.
ClinicalTrials.gov is the most used database
There are many different national and international databases for registering clinical studies. Two thirds of the studies in this follow-up report that they are registered in ClinicalTrials.gov. The second most used database is reported to be the European Clinical Trials Inforamtion System (CTIS), and up until 2022/23, EudraCT was also used, where all clinical trials on medicinal products (intervention studies) shall be registered by law.
The follow-up shows that it is also relatively common to use the Swedish database Researchweb/FoU Sverige. There are also a number of other databases, used by a couple of studies in the follow-up. These include ISRCTN Registry, for example.
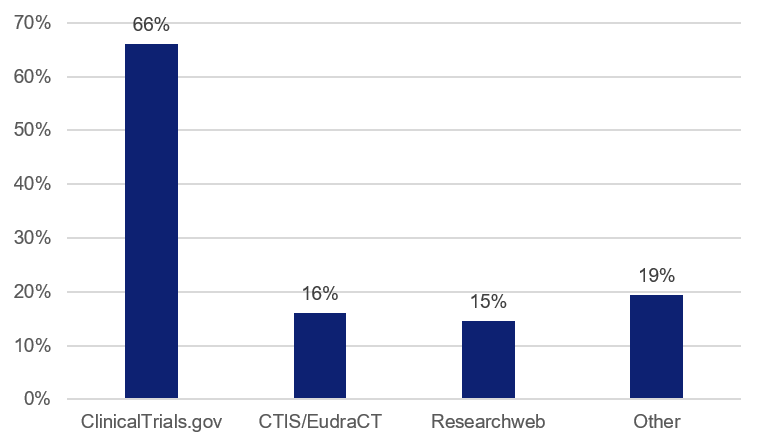
Figure 2. Distribution of the databases used for study registration. The total exceeds 100%, as some studies have been registered in more than one database.
About the follow-up
In total, 90 grants to clinical studies (with project starts up to January 2023) divided up into 16 different administrating organisations were included in the follow-up. The grant recipients had in their applications stated that the research involved a clinical study including humans, and/or biological material from humans.
Seven grants were removed from the summary; five as a reult of the project leaders stating that the grant does not relate to a clinical study, or alternatively that the study will no longer be carried out, and two as a result of lack of response.
The follow-up was carried out between December 20023 and January 2024.
MORE WITHIN THE SAME SUBJECT AREA
-
Activity |
Published 10 April 2024
On 12 of April, there will be an inauguration of one of the new centres of excellence with funding from the Swedish Research Council: Chemical Mechanisms of Life – an interdisciplinary centre for research into the chemistry of life. Mattias Marklund,...
Keywords:
-
Article |
Published 4 April 2024
The Swedish Research Council is funding 15 centres of excellence as from 2024. The centres, which are being built up, shall carry out ground-breaking research and educational activities around a central theme.
-
News |
Published 19 December 2023
The Swedish Research Council and Forte have mapped research in women’s health and diseases. The result is presented in a new report. The report points out knowledge needs linked to women’s health and diseases, and what research initiatives are needed...